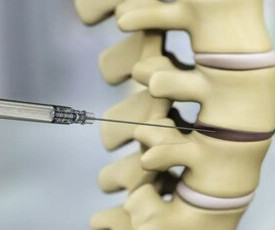
DiscGenics Announces US FDA Approval to Proceed with Phase III Clinical Evaluation of Allogeneic, Injectable Disc Progenitor Cell Therapy for Symptomatic Lumbar Degenerative Disc Disease
OrthoSpineNews
JULY 16, 2024
Receives FDA acceptance on Phase III clinical protocols for parallel pivotal and confirmatory trials Achieves alignment with FDA on Chemistry, Manufacturing, and Controls (CMC) aspects of Phase III program SALT LAKE CITY, July 16, 2024 /PRNewswire/ — DiscGenics, Inc.,



Let's personalize your content